Antiretroviral therapy (ART) for the treatment of human immunodeficiency virus (HIV) infection has improved steadily since the advent of potent combination therapy in 1996. New drugs have been approved that offer new mechanisms of action, improvements in potency and activity even against multidrug-resistant viruses, dosing convenience, and tolerability.
The Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents (the Panel) is a working group of the Office of AIDS Research Advisory Council (OARAC). The primary goal of the Panel is to provide recommendations for HIV care practitioners based on current knowledge of antiretroviral (ARV) drugs used to treat adults and adolescents with HIV infection in the United States. The Panel reviews new evidence and updates recommendations when needed. The primary areas of attention have included baseline assessment, treatment goals, indications for initiation of ART, choice of the initial regimen in ART-naive patients, drugs or combinations to be avoided, management of adverse effects and drug interactions, management of treatment failure, and special ART-related considerations in specific patient populations.
These guidelines generally represent the state of knowledge regarding the use of ARV agents. However, because the science evolves rapidly, the availability of new agents and new clinical data may change therapeutic options and preferences. Information included in these guidelines may not be consistent with approved labeling for the particular products or indications in question, and the terms “safe” and “effective” may not be synonymous with the Food and Drug Administration (FDA)-defined legal standards for product approval. The guidelines are updated frequently by the Panel (current and archived versions of the guidelines are available on the AIDSinfoWeb site at http://www.aidsinfo.nih.gov). However, the guidelines cannot always keep pace with the rapid evolution of new data in this field, and they cannot provide guidance for all patients. Clinicians should exercise clinical judgment in management decisions tailored to unique patient circumstances.
The Panel recognizes the importance of clinical research in generating evidence to address unanswered questions related to the optimal safety and efficacy of ART. The Panel encourages both the development of protocols and patient participation in well-designed, Institutional Review Board (IRB)-approved clinical trials.
国内新增免费hiv药物 2012年美国OARAC成人和青少年HIV抗逆转录病毒医治医治更新攻略
精彩推荐
- 从春晚舞台看中国智造,尊界S800给出中国高端汽车的回答

每年央视春晚都是全民关注的焦点,更是国家形象与产业实力的重要展示舞台,2026年春晚迎来了一位特殊的合作伙伴尊界S800。继2025年春晚完成品牌首次亮相后,尊界S800此番以...详细
- 突破十万交付大关!全新问界M7用硬核实力回应市场认可

在风起云涌的新能源汽车市场,一款车型能否成为真正的标杆,往往并不只取决于华丽的技术参数或前卫的设计语言,而在于它能否真正走进千家万户,赢得用户用选择投出的信任票...详细
- 光影与智慧的双重献礼:解析尊界S800如何借新春OTA重塑豪华出行

在智能汽车不断刷新功能边界的今天,一次真正有意义的OTA升级,应如春风化雨,既能雕琢体验的细微之处,亦能筑牢安全的钢铁长城。岁序更迭,万象更新之际,尊界S800奉上精...详细
- 智能革命,豪华新生!尊界S800以双重价值重塑百万座驾标杆
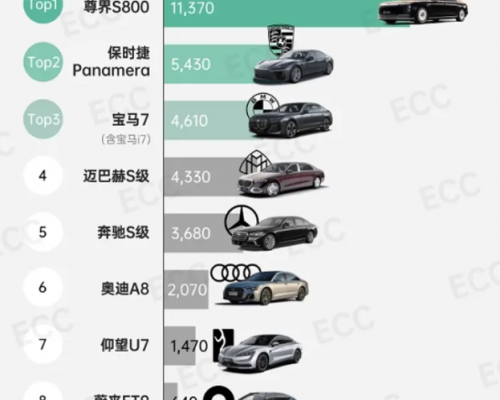
长久以来,百万级豪华车市场犹如一个被精心守护的俱乐部。然而,一款来自中国的座驾尊界S800,正以破竹之势改写规则。它不仅连续五个月稳坐70万元以上细分市场销冠,更以五...详细
本周热门
- 同仁堂健康双十一活动开启 “象食养医”倡导从健康的时候就关注健康

如果你想了解自己身体的秘密,让健康成为日常的生活方式,保持年轻的状态,实现抗衰老,逆生长的美好愿望,那么今年双十一的这场活动你一定不要错过。11月1日,同仁堂健康...详细